CoSo Health to Exclusively Distribute Medinol's EluNIR-PERL Coronary DES in the U.S.
 Medinol Receives FDA Approval for Next Generation EluNIR-PERL™ Drug-Eluting Coronary Stent System
Medinol Receives FDA Approval for Next Generation EluNIR-PERL™ Drug-Eluting Coronary Stent System
CoSo Health to exclusively distribute Medinol's most advanced coronary DES in the U.S.
DENVER and TEL AVIV, Israel, Oct. 24, 2023 /PRNewswire/ -- Medinol today announced United States Food and Drug Administration (FDA) approval for the EluNIR- PERL™ drug-eluting stent (DES) for the treatment of coronary artery disease.
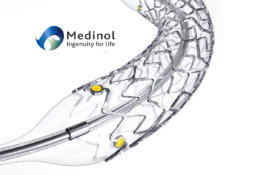
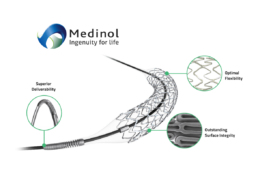
EluNIR-PERL builds upon the proven performance and clinical data of the EluNIR™ DES system. As the latest evolution in the EluNIR DES family, EluNIR-PERL features four radiopaque markers, two at each end of the stent, and a hybrid polymer-metal radiopaque catheter tip. These unique features allow outstanding visualization during PCI procedures whether navigating through complex anatomies or precise placement of the stent.
We are pleased to bring technologies to the U.S. that focus both on benefit to patients as well as unique and meaningful advantages to surgeons, allowing for precise stent placement, shorter procedure times and reduced radiation exposure
Yoram Richter, CEO, Medinol
“We are proud to distribute one of the world's best drug-eluting stents via our intuitive and easy-to-use SaaS+ platform, which reduces layers in the supply chain," said Jae Lee, CEO of CoSo Health.
EluNIR-PERL™ is exclusively available in the United States through CoSo Health, an innovative healthcare supply and logistics company changing the way medical devices are distributed and sold.
About Medinol
At Medinol, we are aggressively changing paradigms in how disease states are diagnosed and treated. Whether designing cutting edge devices for stenting multiple areas of the body, dramatically reducing complications in Structural Heart procedures or providing real time insights into the physiological metrics of the human body through implantable sensors, we boldly reassess current technology and procedures and look years into the future to pioneer new devices to broaden the reach of physicians both physically and geographically. For more information, see www.medinol.com.
Medinol Partners with CoSo Health to Launch the EluNIR™ Drug-Eluting Stent in the U.S.
 Medinol Partners with CoSo Health to Launch the EluNIR™ Drug-Eluting Stent in the U.S.
Medinol Partners with CoSo Health to Launch the EluNIR™ Drug-Eluting Stent in the U.S.
CoSo Health to work with U.S. Endovascular and others to reach the U.S. market
May 23, 2023
DENVER, Colorado - May 23, 2023 /PRNewswire/ CoSo Health today announced signing a U.S. distribution agreement with Medinol for EluNIR™, a new generation, thin-strut drug-eluting stent (DES). Medinol is a global leader in the development of innovative implantable medical devices. U.S. Endovascular sells peripheral and coronary devices nationwide.
CoSo Health is changing the way medical devices are distributed and sold. Through its sales rep-light model, innovative cloud-based logistics platform and customized delivery model, CoSo Health cuts waste from the supply chain.
The announcement comes at a time when hospitals, payers and outpatient facilities are seeking ways to reduce surgical costs.
We are extremely excited to partner with CoSo Health to expand our reach within the U.S. We are both innovators – Medinol with world-class implantable device technology and CoSo Health with logistics and distribution. Together, we are disrupting the distribution of drug-eluting stents through a direct-to-end user model that brings tremendous economic value to purchasers.
Jeff Roach, Chief Commercial Officer, Medinol
The EluNIR DES offers excellent clinical outcomes and an outstanding safety profile. Its best-in-class metal-to-artery ratio and ultra-durable polymer coating, along with a unique delivery system, combine to provide a new standard in treatment capabilities. Successive generations of EluNIR are coming to the global market soon.
“EluNIR is a tremendous option for physicians seeking a next-generation DES,” said Jae Lee, CEO of CoSo Health. “For healthcare buyers, we see a great opportunity to obtain one of the world’s best drug-eluting stents through a model that reduces supply chain costs.”
About Medinol
At Medinol, we are aggressively changing paradigms in how disease states are diagnosed and treated. Whether designing cutting edge devices for stenting multiple areas of the body, dramatically reducing complications in Structural Heart procedures or providing real time insights into the physiological metrics of the human body through implantable sensors, we boldly reassess current technology and procedures and look years into the future to pioneer new devices to broaden the reach of physicians both physically and geographically. For more information, see www.medinol.com.